Like many businesses, LaunchWorks was thrown for a loop when the COVID-19 pandemic hit, but not in the same way as some other manufacturers. The company was already on a strong growth trajectory for 2020, but when shelter-in-place mandates were announced, access to doctors’ offices limited the activity of most of the existing customer base. The company was able to harness its strengths to quickly pivot by promoting and expanding COVID-19 test kit manufacturing capabilities. As a result, LaunchWorks anticipates tripling its size this year. Automation is a key factor in scaling up to serve clients.
Expanding that quickly to help meet the country’s public health needs is not a simple task. The company focused on a combination of long-term industry supplier relationships, innovative thinking and automation solutions. LaunchWorks already utilized automation, but adding new functionality and additional capacity for the COVID-19 efforts helped move from producing 75,000 transport media tubes a week to an anticipated 1 million per week.
From contract manufacturing to development
Twelve years ago, LaunchWorks began as a contract manufacturing facility for diagnostics. Today the company offers design and development services as well. Given the history of producing kits for molecular diagnostics, LaunchWorks’ manufacturing services attracted current and new clients needing support with COVID-19 testing kit development and production, from an organization operating under GMP ISO-13485:2016 authority. In addition, when the Centers for Disease Control (CDC) shared the viral transport media recipe this spring, the company began exploring the best ways to produce the media to assist in the testing efforts as a contract manufacturer for laboratories. Today LaunchWorks produces PCR-based diagnostic kits as well as serology ELISA kits. For some clients, the company handles the process from end-to-end. For others, it’s just formulation, filling and/or fulfillment.
Growth through automation
The country is experiencing testing availability challenges, with not enough tests and/or supplies for those who want or need them. Test numbers are rising, though, as testing kits ramp up and more providers can offer them. From July 1-8, an average of 641,635 tests were used per day in the U.S. Compare that to May, when testing numbers ranged from 247,084 to 487,229 per day. And that’s up from April, where daily testing numbers ranged from 108,521 to 239,407 daily.
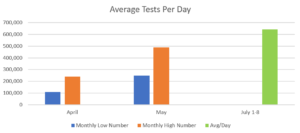
The growth seen in testing capacity is similar to the growth in LaunchWorks’ production. As the unemployment rate rose and college kids returned home from school early, the company offered summer jobs to life science workers, graduating college students whose career plans were paused by the pandemic, and enrolled students in scientific fields who were unable to complete planned internships or other summer work in the Boston area. In June LaunchWorks scaled 2X by adding a 2nd shift. Now two new automation machines are operating, with a third in transit. In July the company scaled another 2X due to expanded automation capacity. With additional machines arriving in the next couple of months, the capacity will again double. As the country is on target to test more than 4 million people a week, LaunchWorks will be able to supply 10% of those sampling tools. With all future machines in place the company will be able to produce 1 million tubes a week. The scaling aligns with the country’s need. The Edmond J. Safra Center for Ethics at Harvard University anticipates needing 5 million tests a day for a safe social reopening, increasing to 20 million tests per day to fully remobilize the economy. That’s a lot of testing.
Manual versus automated filling
Prior to the automation equipment’s arrival, employees manually dispensed the fluid into containers. The tubes were then manually capped. One new automated filler can complete the same production numbers while reducing the labor hours by a factor of 9X. Additionally, filling and capping tubes 8 hours a day is repetitive and lends itself to workplace safety issues. LaunchWorks redistributed the current workforce to production of other kits while increasing COVID-19 testing supplies for the country.
The automation also positively affects the quality process. The company works in a regulated environment with many quality checks. While there is no difference in quality between hand-filled and machine-filled test kits, the quality inspection process differs. When filled manually, 100% of the vials need inspection to ensure the fluid level is consistent. With automation, there are a predetermined number of checks each shift. LaunchWorks’ automated labeling and inline quality inspecting technology allows for periodic visual inspections of the labeling as well, with no need to visually review every label.
Shifting capacity
Once the pandemic ends, the filling and capping machines will be easily redeployed for different formulations, allowing the company to reduce the cost of manufacturing for future customers and supporting LaunchWorks’ increase in ability to scale other projects. Automation transformed the life sciences manufacturing industry over the past three decades, and it’s doing the same to help the country now with COVID-19 test kit production.
Please reach out to the company at www.launchworksCDMO.com to learn more about the automation program and how LaunchWorks can help your company with its life sciences contract and development manufacturing needs.